Project Leader : Akiko Hayashi-Takagi,
Multi-scale Biological Psychiatry, RIKEN CBS

Project Leader : Akiko Hayashi-Takagi,
Multi-scale Biological Psychiatry, RIKEN CBS
Despite extensive recent efforts, the pathogenesis of psychiatric disorders remains poorly understood, mainly because their pathophysiology is a synergistic interaction between multiple genes variants and environmental factors. Thus, what we recently know as contributory factors for the diseases is the susceptible gene variants (Molecular layer), synaptopathy (Subcellular and Cell layer), alteration in neuronal circuits (Circuit layer), conceivably resulting in the behavioral manifestations (Individual layer). However, the understanding of each layer has been limited within a single layer, which hinders the integrative and causal mechanistic understanding of behaviors. Probably, each layer can affect one another, macroscale to the mesoscale and then to the microscale layer or vice versa. Thus, we deal with phenomena of intricate complexity of psychiatric disorders that are governed by various mechanisms integrated across multiscale layers.
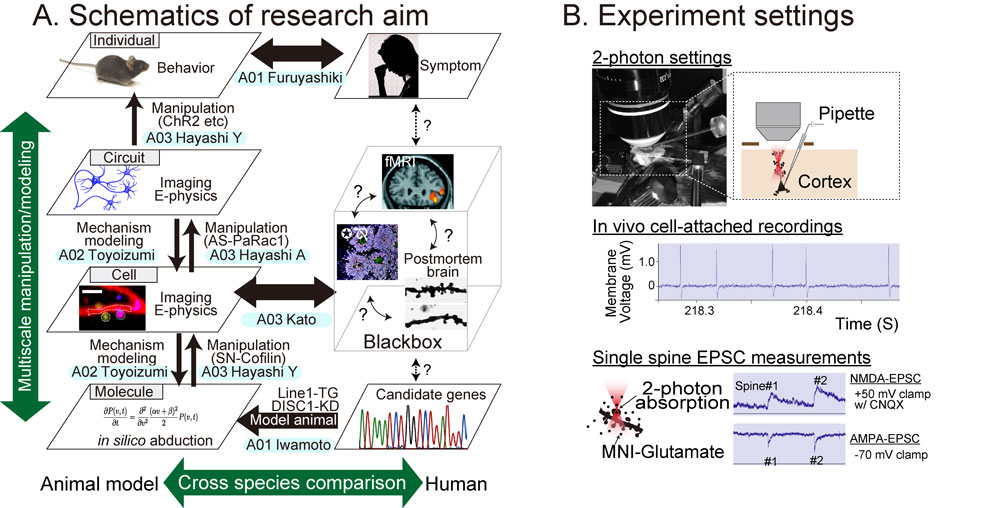
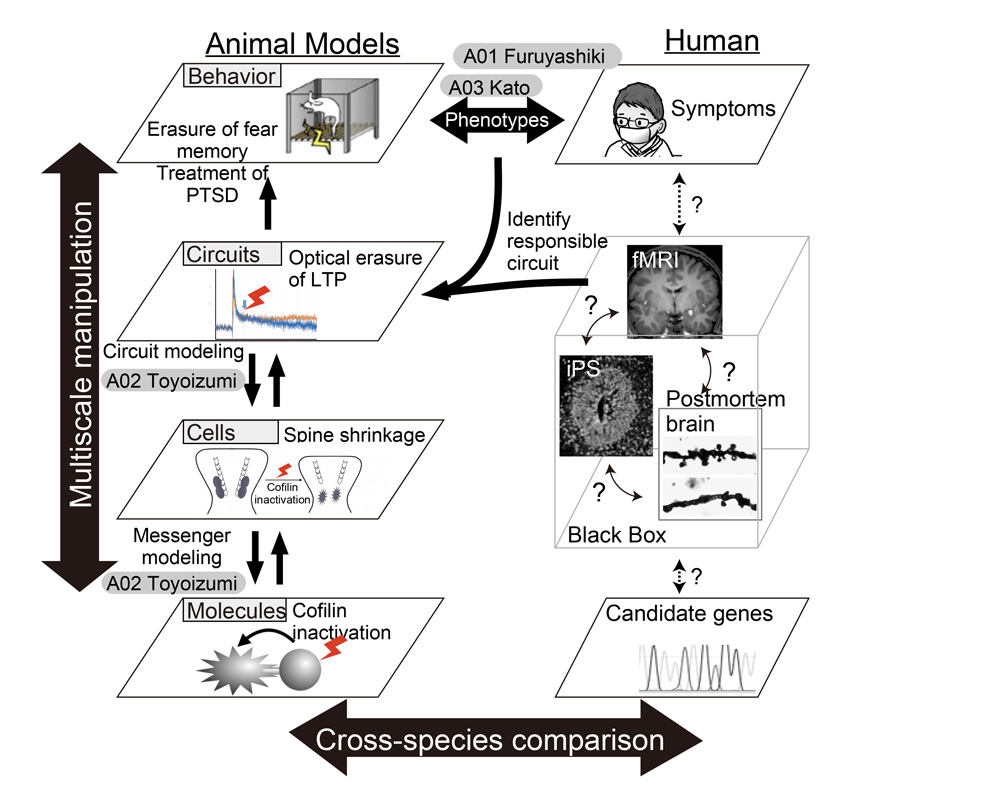
In this study, we aim at a constructive understanding of multiscale hierarchical nature of psychiatric disorders with use of recently available state-of-art techniques (Figure 1): hypothesis-free and comprehensive data sampling, which can handle big data from multiscale layers ranging from molecules/ synapses/neurons/circuit (A01: Data-driven approach).
Second, big data from A01 team will be analyzed and subject to the construction of mathematical models (A02: Abduction approach) so that the candidate of responsible molecule or mechanism will be identified. The abducted hypothesis by A02 will be examined by manipulative experiments (A03: Hypothesis-driven approach). Besides, by using induced pluripotent stem (iPS) cells derived from patients with psychiatric disorders, neural cells and cerebral organoids will be generated, and cellular pathology underlying mental disorders will be reconstructed.
Figure 1
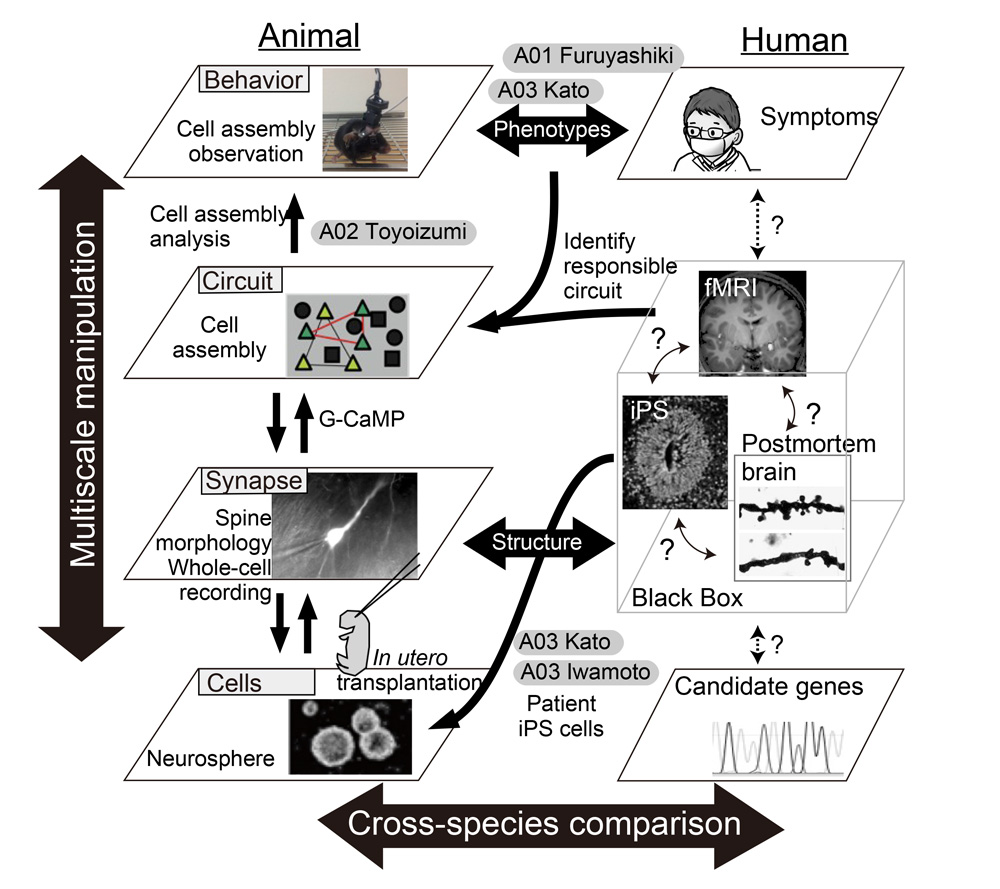
Through the series of studies as described above, a mathematical model of psychiatric disorders that incorporate multiple layer facets including molecular, cellular, circuit and behavioral levels, will be constructed, and thereby we will aim at a constructive understanding of the multiscale phenomena of psychiatric disorders (Figure 2). One of the goals of neuroscience research is to elucidate how specific neuronal circuits are altered in the disease state. Findings based on our strategy that would causally identify the contributory factors for the disease will provide the knowledge necessary to establish circuit-centric therapeutics as well as the rationale molecular (and chemistry) based drug designs.
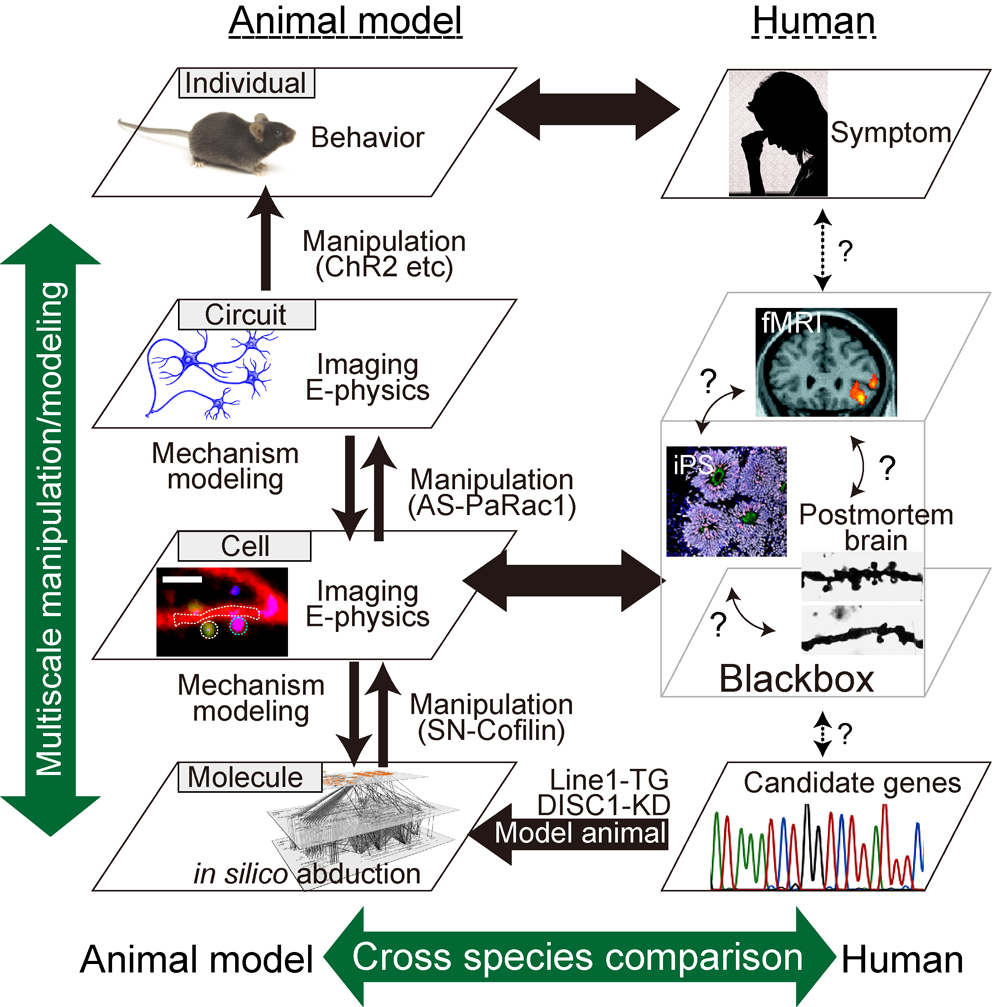
Figure 2
Psychiatric disorders, Multiscale, Constructive understanding, Optical manipulation, Modeling, Transomics
FY2018-2022

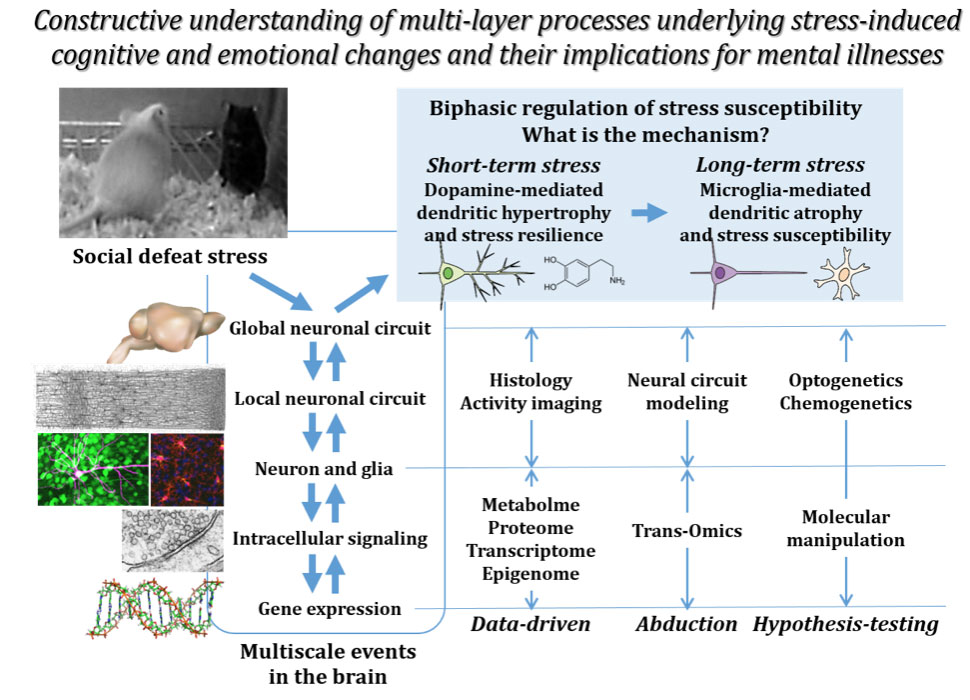
Stress from social environments has various behavioral consequences, depending on the specific conditions. Brief exposure to stress induces adaptive behavioral responses, whereas prolonged stress causes negative behavioral consequences, including depression and anxiety, and can be a risk factor for mental illnesses. Previous studies have shown that stressful stimuli alter functions and structures of neurons in stress-related brain areas, such as the medial prefrontal cortex, thereby regulating stress susceptibility. Stress induces rapid changes in intracellular signaling and metabolism, and slow changes in gene expression in specific brain regions and cell types. Consequently, functional and structural changes of neurons occur and result in alterations in their specific inputs and outputs, which in turn affects local and global neuronal networks. Therefore, regulation of stress susceptibility is a multiscale phenomenon ranging from the molecular, cellular, neuronal circuit, and behavioral levels. However, causal relationships linking these multiple levels remain to be elucidated.
In this research, we will examine stress-induced multi-level molecular changes in transcriptional and epigenetic regulation, intracellular signaling, and metabolic pathways in specific brain regions and cell types. We will also visualize functional and structural changes of neurons at the synaptic, local-circuit, and global-circuit levels. We will integrate these changes to forge an in silico stress model. Cultured cells will be employed to examine multi-level molecular changes induced by stress-related stimuli, which will be compared with those in vivo. Molecular and opto/chemogenetic manipulations will be used to test the validity of the in silico stress model as well as its relevance to stress susceptibility, and to manipulate stress susceptibility in normal animals and correct it in animal models of mental illness. Collectively, we aim to elucidate a multiscale phenomenon underlying the regulation of stress susceptibility and to develop methods to manipulate stress susceptibility. This approach will promote our understanding of stress-related abnormalities, and may contribute to therapeutic development for mental illnesses.

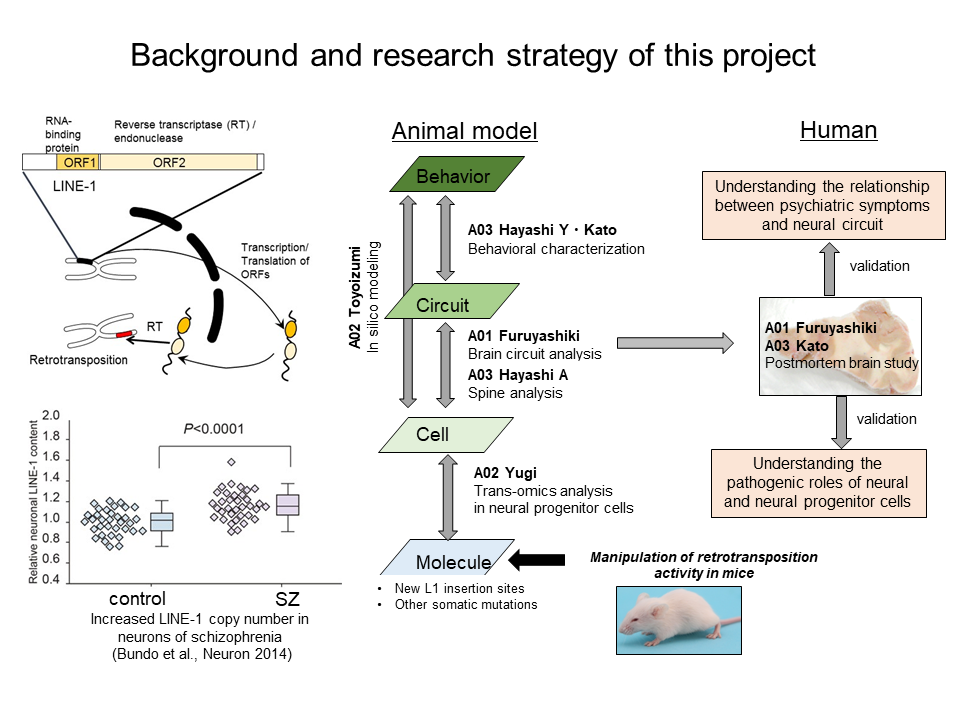
Schizophrenia, one of major psychiatric disorders, is thought to be caused by the complicated interaction between genetic and environmental factors. However, genetic factors with large effective sizes have not been identified. Accumulating evidence suggest that various somatic mutations in the neuronal genome have important roles in the normal physiology of brain. It is possible that altered frequency and pattern of somatic mutations are closely related to the pathogenesis and pathophysiology of psychiatric disorders. In particular, retrotransposon LINE-1 is known to be activated in neural progenitor cells, and somatic new retrotransposition is occurring at this stage. We found that the copy number of LINE-1 was increased in the postmortem brains of patients with schizophrenia, and new insertions have occurred in genes important for neuronal functions. However, molecular mechanism of increased LINE-1 copy number and causal relationship with schizophrenia remained unclear. In this project, we try to clarify what kind of brain neurons, neural circuits, and brain regions are affected by retrotransposition using the multiscale approaches. We then revalidate by genomic analysis of target brain cells, circuit and regions using postmortem brain samples.

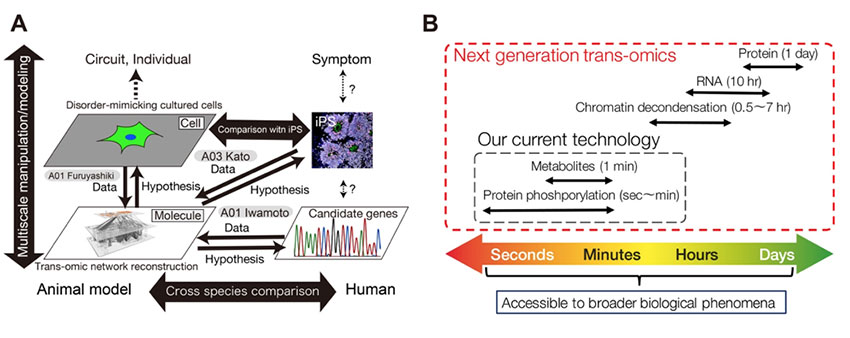
We will develop next-generation trans-omics technologies that allow characterization of psychiatric disorders as multilayered molecular networks. Current trans-omics technologies are capable of reconstruction of networks that span across "fast" omics layers such as metabolome and phosphoproteome that change in minutes scale. However, “slow” omics layers such as those related to gene expression, on which molecular basis of psychiatric disorders largely rely, cannot be handled with current trans-omics technologies because of the difference of time scale. Therefore, in this study plan, we will develop next-generation trans-omics technologies that connect the omics layers of metabolism and gene expression beyond the difference of time scale. With the next-generation technologies, molecular basis of various diseases and biological phenomena including psychiatric disorders, which cannot be handled with the current trans-omics technologies, can be characterized in terms of network reconstruction. In cooperation with other groups, we apply the next-generation technologies to network reconstruction of various nervous system cells such as patient-derived iPS cells and cultured neural progenitor cells. Then, we provide hypotheses on molecular basis (i.e. reconstructed multi-layered networks) behind the functional degeneration of the nervous system cells to other groups. Furthermore, we identify the core part of the multi-layered network, and find responsible molecule candidates by analyzing the mathematical model in silico. Such trans-omics and systems biology methods will allow comprehensive understanding multiscale structure of psychiatric disorders from nano (genes / molecules) and micro (synapse / cell), the main goal of this research area "stratagical structural understanding of psychiatric conditions".

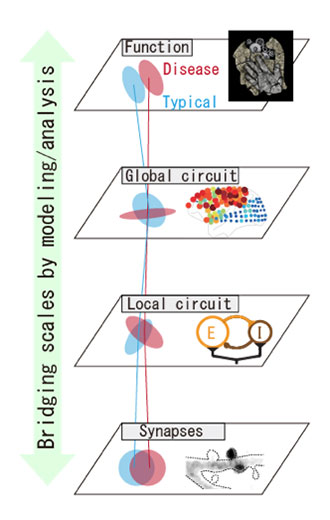
We employ theoretical and modeling approaches to understand multiscale phenomena involved in mental diseases. In particular, synaptic abnormalities are commonly observed in multiple model animals of mental disease. We utilize computational modeling to bridge synaptic abnormalities, circuit dynamics, and the behavioral output resulting from them.
Recent studies have identified that spines undergo constant remodeling even in the absence of spiking and calcium activity of neurons, and that a few model animals of mental disease exhibit abnormal intrinsic spine dynamics. However, it is unknown how the intrinsic spine dynamics interact with Hebbian synaptic plasticity, which is thought to be the mechanism of learning and memory. It is often assumed that Hebbian synaptic plasticity forms a cell assembly, a mutually interacting group of neurons that encodes memory. However, in recurrently connected networks with pure Hebbian plasticity, cell assemblies typically diverge or fade under ongoing changes of synaptic strength. Previously proposed mechanisms for stabilizing cell assemblies do not robustly reproduce the experimentally reported unimodal and long-tailed distribution of synaptic strength. Here, we study the role of both normal and abnormal intrinsic spine dynamics in learning and memory. Specifically, we explore how Hebbian plasticity with experimentally observed intrinsic spine dynamics affects the stability of cell assemblies, the distribution of spine volume, and learning performance.

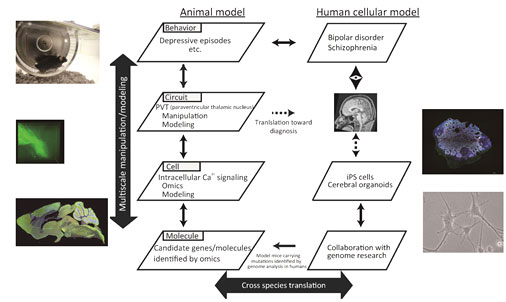
Bipolar disorder and schizophrenia are two major mental disorders that cause severe social burden, and there is urgent need to clarify their etiology. These diseases share common features, such as role of genetic factors and environmental factors during developmental period. Indeed, individual genes and environmental factors that confer a risk are shared by these disorders. Although a number of hypotheses have been proposed in these disorders, they have focused on a single layer of pathophysiological architecture, and understanding of each layer such as molecular, cellular, neural circuit and brain levels, is not connected with that of other layers, which hampers constructive and integrative understanding of these mental disorders as a multiscale phenomenon.
In this study, we aim at constructive understanding of pathophysiology of mental disorders by integrating these layers. For this purpose, the role of genes identified by genetic analysis of families of bipolar disorder and schizophrenia as well as molecules identified by omics analysis of postmortem brain samples, in intracellular and inter-cellular signaling abnormalities, will be analyzed and subject to the construction of mathematical models. Using animal models of the candidate genes, responsible neural circuit will be identified by behavioral and anatomical analyses. Within that neural circuit, responsible cell types will be identified using omics analysis and the mechanism for the emergence of behavioral changes will be pursued by manipulation of specific neural circuit and by employing mathematical modeling of the responsible neural circuit.
Using induced pluripotent stem (iPS) cells derived from patients with mental disorders, neural cells and cerebral organoids will be generated and cellular pathology underlying mental disorders will be studied using omics analyses. Through these series of studies, mathematical model of mental disorders that incorporate multiple layer facets including molecular, cellular, circuit and behavioral levels, will be constructed and thereby we will aim at constructive understanding of the multiscale phenomena of bipolar disorder and schizophrenia.

Synaptic transmission is the basic element of information processing and storage in the brain. Furthermore, abnormality of synaptic function is implicated not only in memory and learning disorders such as developmental disorder, posttraumatic stress disorder (PTSD), dementia, Alzheimer's disease, but also on the pathogenesis of schizophrenia and depression. However, it has not been possible to identify the synaptic abnormality, responsible for a certain pathological situation. In this study, we will establish a system to manipulate synaptic plasticity based on our knowledge and techniques on the molecular mechanism of hippocampal synaptic plasticity. We will further employ techniques on iPS cells, molecular genetics, animal behavior experiments. By controlling the neural circuit and behavioral at the multiple scale ranging from the molecule to behavior, we will attempt to understand psychiatric disease at multiple laysers. We set the following Specific Aim.

Various lines of evidence, including human genetics, brain imaging, and postmortem brain studies, have repeatedly suggested that disturbances in neuronal connectivity, synaptopathy, underlie a variety of psychiatric disorders. However, it is not yet known whether synaptopathy is an underlying mechanism of disease or a secondary consequence. Thus, we performed a longitudinal in vivo 2-photon imaging analysis of the brain of a schizophrenia model (DISC1 knockdown mice). Our previous work demonstrated that Disc1 knockdown mice exhibited a decrease in the density of dendritic spines, where the majority of excitatory synapses are formed. Furthermore, we found a significantly greater number of large dendritic spines in the model mice compared to wild-type mice. The presence of the large spines in the schizophrenia mice model mirrors findings from another schizophrenia mice model, calcineurin knockout mice. It is well-known that there is a strong correlation between spine head size and its synaptic efficacy, whereby the large spines can generate a larger synaptic current. This led us to hypothesize that large spines can affect the dendritic computation, causally resulting in subsequent behavioral alterations. To test this hypothesis, in our current project, we would use a multiscale analysis that consists of an electrophysiological method and Ca2+ imaging to visualize the synaptic input (synaptic level), dendritic event (dendritic level), action potential (cell level), and behavioral manifestations (individual level). In addition, with use of in vivo optical and in silico manipulation of the spines in the model animals, as well as in vitro validation of these findings in patient-derived iPS cells, we will causally examine what kind of synaptic pathology would underlie the pathology of disorders at the multiscale level.